Osmosis Definition –
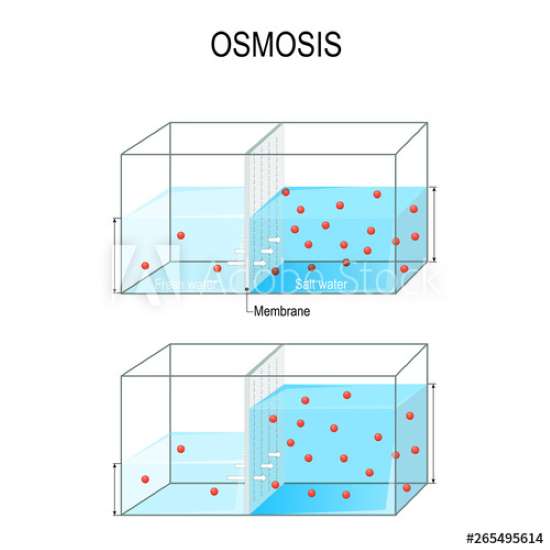
So, what is osmosis? In simple words, osmosis is the movement of water through a special mixture of solutes like salt particles within the solvent. In osmosis, water moves from an area of higher concentration to lower concentration through a selectively permeable membrane
A selectively permeable membrane is known as a cell membrane. These cell membranes have small openings that let the water molecules pass through them. But it won’t allow larger particles like salt molecules to move freely through it.
Learning courses for your kids! Get free trial here
Osmosis allows water to pass through cell membranes. The transfer of water from a dilute solution to a concentrated solution over a partly permeable membrane is defined as osmosis.
To further explain the concept of osmosis for children, they must first understand what lower and higher concentrations of water are.
We have to remember that water has a natural propensity to flow from higher to lower concentration areas. The increased concentration of water contains no or fewer solutes.
Types of Osmosis
There are two forms of osmosis:
Endosmosis
When a material is immersed in a hypotonic solution, the solvent molecules travel into the cell, causing the cell to become turgid or undergo de-plasmolysed. This is referred to as endosmosis.
Exosmosis
When a material is put in a hypertonic solution, the solvent molecules travel outside the cell, causing the cell to become flaccid or plasmolysed. This is known as exosmosis.
What is Osmosis in Biology for Kids: Examples
Let’s look at one example to understand this concept better.
Consider a glass tank with a semipermeable membrane in the centre that allows solvents such as water to pass through but not bigger particles of solutes, such as salt and sugar. Now, on both sides of the tank, fill it with fresh water. Name them A and B. You will see that the water molecules are in motion. We can see water molecules moving in the tank, but the net movement on both sides is zero.
It denotes that there is no general shift in the flow direction. Now it’s time to add some salt to side B. This membrane can’t let salt particles transmit since they’re too big. As a result, they will remain on the same side. The question is, will the water flow from one way to another?
It will change sides from A to B. Side A has more solutes, and more solutes means less water concentration.
Also Read: Educational Activities for Preschoolers: Let Your Kids Have Fun While Learning
Examples
There are many more examples of osmosis to understand the concept better.
Take some raisins for demonstration. Raisins have relatively low water content. Put some raisins in the water. Water passes across a semipermeable membrane from a greater concentration to a lower concentration region. Raisins will swell as a result of absorbing water. Water begins to seep into the raisins, causing them to expand.

Now, let’s put these inflated raisins in sugar syrup. Compared to the sugar syrup, the raisins now have a greater water concentration. As a result, the water from the raisins will flow to the sugar syrup. The raisins will also return to their normal size and shape.
Another Experiment
Let’s try another experiment to learn more about how water moves during osmosis.
Take two eggs and three beakers, each containing diluted hydrochloric acid, concentrated salt water, and regular water. Wait for a few days after deshelling the eggs by dissolving them in weak hydrochloric acid. Pour one de-shelled egg into the water and the other into a strong salt solution.

Allow the beakers to sit undisturbed for a while and observe. The egg in the water swells as water runs through it due to osmosis. This occurs because the egg in the beaker has less water content. The egg in the salt solution, on the other hand, shrinks. Because saltwater has a lower water content than freshwater, water flows out of the egg. This demonstrates two forms of osmosis.
Osmosis Facts for Kids
Here are some interesting facts about osmosis:
# Osmosis can be observed in the working of specific organs, notably the kidneys. The kidney’s job is to filter waste items from the bloodstream and expel them in the form of urine. More than a million microfilters called nephrons are found in each kidney, allowing microscopic particles like water, glucose, urea, and ions to flow through while keeping blood molecules out.
After this filtration, the kidneys must reabsorb enough water to keep the blood plasma in a healthy equilibrium through osmosis.
# Hypotonic solutions have a lower concentration of solutes outside the cell; hence, water permeates the cell, which gains volume. A hypertonic has a higher solute concentration than inside the cell, and the solutes cannot cross the membrane.
# A partly permeable membrane allows water to pass through it. Larger molecules, such as sugar are unable to pass through it.
# Water travels from a dilute solution (greater water concentration/ lower solute concentration) to a concentrated solution (lower water concentration/ higher solute concentration).
# When too much water enters a cell through osmosis in living things, it expands. Animal cells are prone to rupture due to heavy expansion.
Learning courses for your kids! Get free trial here
What is the Importance of Osmosis?
Osmosis is crucial to living creatures for multiple reasons.
#1. The Significance to Plant Cells

Plants need to get vital elements into and out of their cells. The process of osmosis occurs whenever you water the plants. Osmosis allows important nutrients to get through while wastage dissolved in water is removed. To operate effectively, cells must maintain a water balance.
#2. Important for Human Beings
It is also necessary for the human body to function properly. Sweat contains water and ions, which are lost when you exercise. It’s critical to replenish them because osmosis can harm cells if too much or too little water is taken into them. Osmosis regulates the healthy functioning of the kidneys in humans.
#3. Significant to Aquatic Lives
Fishes that live in freshwater do not have to drink water because the water flows into them through osmosis.
Osmosis for Children
The concept of osmosis is essential to understand life processes in the plant and animal kingdom. It is vital to teaching young children about the meaning, functioning, and working of osmosis in exciting ways. The Real schoolOf Montessori is working in this direction by making learning easier, interesting, and fun for kids through practical examples.
Conclusion
The Real School Of Montessori has designed the right curriculum to focus on every aspect of learning easily. The core team at the Real School Of Montessori are experts with entrepreneurial experience in running education programs in over 300 schools. The research has evolved into a robust, transformational program and its honesty and relevance to real problems and the real world. Please stay connected with us to understand more such significant topics interestingly.
Read well and learn more!
Also Read: Educational Activities for Preschoolers: Let Your Kids Have Fun While Learning